At a glance
- This page provides instructions for U.S. Department of Agriculture (USDA)-accredited veterinarians completing the Certification of U.S.-issued Rabies Vaccination form.
- Each U.S.-vaccinated dog that has been in a high-risk country for dog rabies in the past 6 months must have a valid Certification of U.S.-issued Rabies Vaccination form to re-enter the United States.
- The form must be completed by a USDA-accredited veterinarian before the dog departs the United States.
- The form can be used multiple times for entry into the United States and is valid until the rabies vaccination listed on the form expires (either 1 or 3 years).
Overview
The Certification of U.S.-issued Rabies Vaccination (For Live Dog Re-Entry into the United States) form is required for the importation (re-entry) of U.S.-vaccinated dogs that have been in countries with high risk of dog-maintained rabies virus variants (DMRVV high-risk countries) within 6 months before re-entry into the United States.
If a dog was issued a USDA-endorsed export health certificate no later than July 31, 2025, that certificate can be used in place of the Certification of U.S.-issued Rabies Vaccination form for re-entry to the United States as long as it:
- Was digitally endorsed by USDA,
- Includes the dog's age (at least 6 months),
- Includes the dog's microchip number, and
- Includes the dog's current and valid (not expired) U.S.-issued rabies vaccination information.
Either certificate may be used for multiple re-entries until the vaccination listed on the certificate expires (1 or 3 years). The rabies vaccination must have been administered after the dog's microchip was inserted. Export health certificates issued after July 31, 2025 cannot be used for re-entry to the United States.
The Certification of U.S.-issued Rabies Vaccination form may also be used by those re-entering the United States with dogs that have been only in DMRVV-free and low-risk countries in the previous 6 months. Please note the use of this form is not required for these dogs.
Instructions for completing the form
This form is valid for one dog only.
This form can be used for multiple re-entries into the United States as long as the rabies vaccination is valid (not expired).
Form requirements
For the form to be considered valid, all the following requirements must be met:
- The dog must have been vaccinated in the United States using a USDA-licensed rabies vaccine.
- The form must be completed by the USDA-accredited veterinarian that vaccinated the dog in the United States. In the event the USDA-accredited veterinarian that vaccinated the dog is unavailable or if the veterinarian is not a USDA-accredited veterinarian, a USDA-accredited veterinarian may certify the form if:
- A valid, in-person, veterinary-client-patient relationship (VCPR) as defined by the American Veterinary Medical Association exists with at least one veterinarian currently in the practice in which the USDA-accredited veterinarian practices medicine; and
- The dog's information (breed, sex, age, microchip) and vaccination history can be verified using the practice's existing medical records. Vaccinations administered at other clinics may be added to the practice's medical records if all required information (including microchip) is contained on those records.
- The form must be completed at least 28 days after the dog's initial rabies vaccine administration or 28 days after booster vaccine administration if the dog's previous rabies vaccination had expired prior to receiving the booster.
- The veterinarian must submit the form to USDA for endorsement before the dog departs the United States. The form must be endorsed by USDA before it can be used for the dog to re-enter the United States.
Rabies vaccine requirements
Dogs must receive their initial rabies vaccination on or after 12 weeks (84 days) of age or in accordance with manufacturer recommendations if licensed for use in older dogs (i.e., 16 weeks of age or older). Vaccines administered prior to 12 weeks of age will not be accepted, regardless of manufacturer product licensing. The initial rabies vaccine must be administered at least 28 days before a USDA-accredited veterinarian may issue this form.
Vaccines will be considered valid for a period of 1 or 3 years depending on manufacturer guidelines. Booster vaccines are considered immediately valid in dogs over 15 months of age provided the dog has received at least 1 previous rabies vaccine administered on or after 12 weeks of age. If there is a lapse in vaccination coverage, the next vaccination will be considered an initial vaccination valid for 1 year, which must be administered at least 28 days before this form may be issued.
If a dog received previous rabies vaccines but did not have a microchip at the time of vaccination:
- The veterinarian must microchip the dog and administer a 1-year rabies vaccine.
- Rabies vaccinations administered prior to the microchip implantation date are invalid because they cannot be verified.
- Future rabies vaccinations may be valid for 3 years, but the initial vaccine administered after microchip implantation is only valid for 1 year.
- Do not enter any rabies vaccination information in the Veterinary Export Health Certification System (VEHCS) for vaccines administered prior to the microchip implantation date.
- The rabies vaccine must be given at least 28 days prior to issuance of this form (the Certification of U.S.-issued Rabies Vaccination form).
Requirement for USDA endorsement
The USDA-accredited veterinarian must submit this form electronically to USDA's Animal and Plant Health Inspection Service (APHIS) Veterinary Services (VS) for endorsement via the Veterinary Export Health Certification System (VEHCS system). CDC will only accept digital USDA APHIS VS endorsement through VEHCS.
The veterinarian must submit the form to USDA for endorsement before the dog departs the United States. The form must be endorsed by USDA before it can be used for the dog to re-enter the United States.
There will be a VS User Fee associated with endorsement of the certificate; certificates will not be processed until payment is received.
VEHCS instructions
To complete the Certification of U.S.-issued Rabies Vaccination form in VEHCS:
- Select "New Certificate"
- Destination Country: Choose United States
- Commodity Type: Dogs
- Intended Use: Pet
- Type of Admission: Choose a value from the dropdown
In the form, enter the following information:
Place of origin
List the name, address, and phone number of the consignor (shipper) of the dog. List physical address. P.O. boxes are not permitted.
Inspection date
Enter date that the USDA-accredited veterinarian examined the dog.
If the USDA-accredited veterinarian did not examine the dog, then enter the date the dog was last examined by a veterinarian in the clinic based on the clinic's existing medical records.
Consignor
Select "add" to enter the name, address, phone number and email address of the consignor (shipper) of the dog a second time.
List physical address. P.O. boxes are not permitted.
If the consignor lives overseas, you should list the foreign home address in this section.
Select “Save & Back.” Select the consignor from the consignor drop-down list.
Consignee
Select "add" to enter the name, U.S. address, U.S. phone number and email address of the consignee (owner) of the dog. This individual may also be the consignor.
List physical U.S. address where dog will be located upon return to the United States. P.O. Boxes are not permitted.
Select “Save & Back.” Select the consignee from the consignee drop-down list.
U.S. port of departure
List intended port of re-entry when arriving back in the United States.
Estimated date of shipment
Enter estimated date of travel out of the United States.
Means of transport
Choose the expected means of transport when arriving back in the United States
Number of containers
Leave blank
Container description
Leave blank
Identification/seal numbers
Leave blank
Description of commodity
Enter 1 for number of rows.
Enter name of dog, microchip number*, microchip implant date±, breed, date of birth, sex, and color of dog.
*Microchip is required for re-entry into the United States. The microchip must be implanted on or before the date of the most recent rabies. Rabies vaccines administered prior to the microchip implantation date are invalid.
±If the implant date is unknown, input the earliest date when the microchip is documented in the dog's medical or vaccination records.
Total quantity
Enter 1
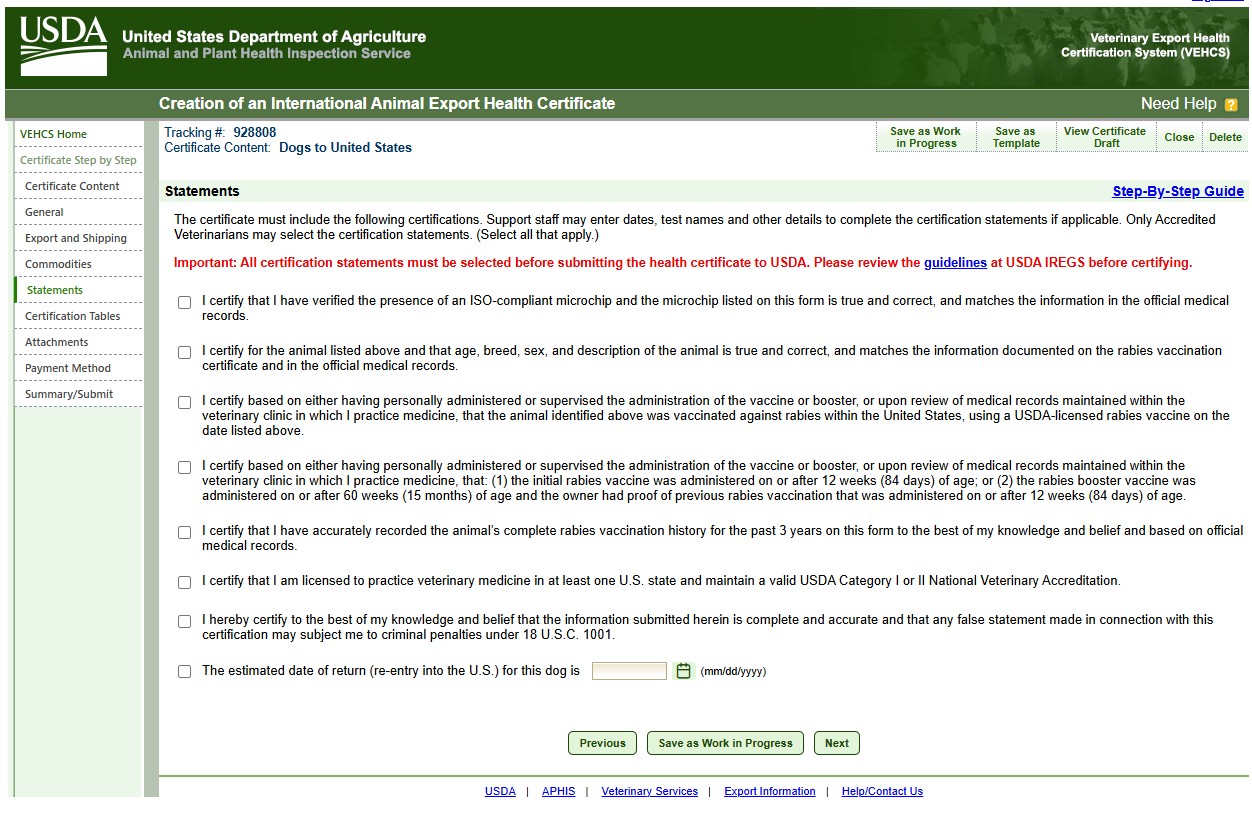
Certification statements
Read and verify all the statements before certifying the form. The form may be completed either based on direct examination of the animal or based on official medical records maintained by the vaccinating clinic in the United States.
Enter the estimated date of return. USDA-accredited veterinarians are not certifying the estimated date of return as the date of return may change and this form can be used for multiple entries as long as the rabies vaccine is valid (not expired). Please inform the owner the date of return must be before the expiration date of the dog's current rabies vaccination information listed on this form or this form will be invalid for re-entry.
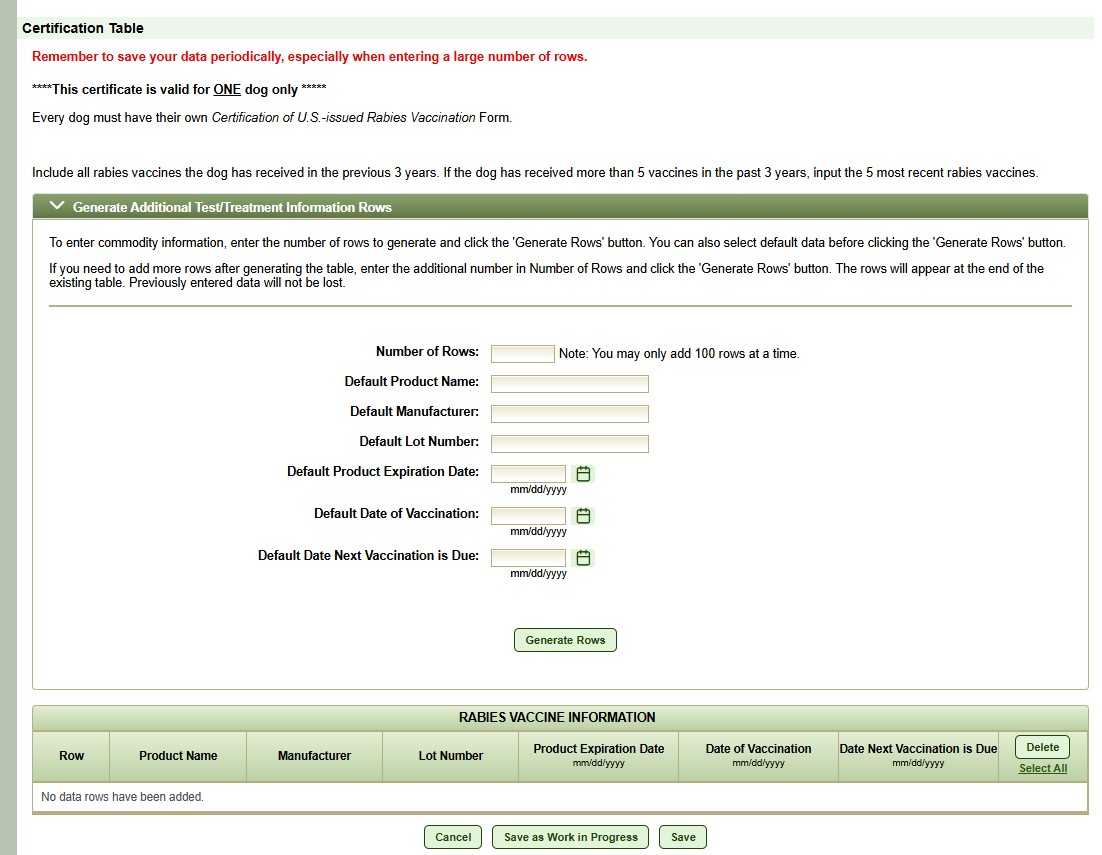
Rabies vaccine information
Veterinarians may include all U.S.-issued rabies vaccines the dog has received in the previous 3 years after the microchip was implanted (if the vaccination history can be verified using the practice's existing medical records). If the dog has received more than 5 vaccines in the past 3 years, input the 5 most recent rabies vaccines.
Enter product name, vaccine manufacturer, lot number, vaccine product expiration date, date the rabies vaccine was administered, and date the next rabies vaccine is due.
For each row of vaccine data, each column must be completed, or the document will be invalid. If the vaccine product expiration date for the most recent vaccination is unknown, CDC will not accept the vaccine record, and the dog must be revaccinated prior to leaving the United States.
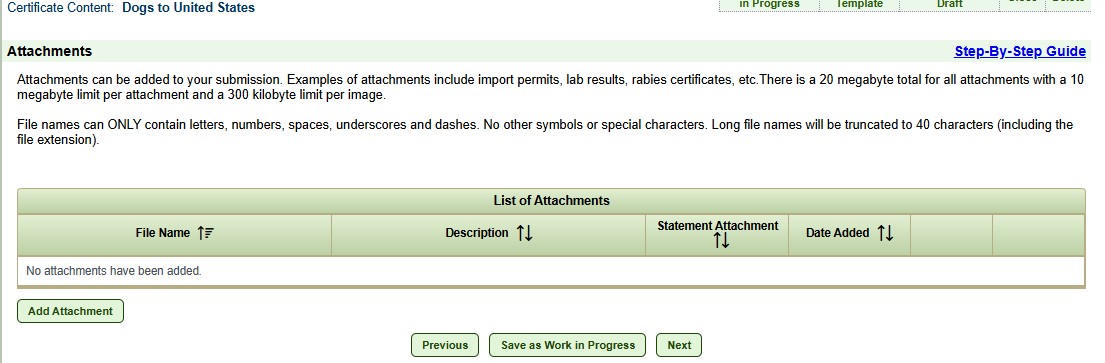
A U.S.-issued rabies vaccination certificate must be uploaded into VEHCS for each vaccine entered into the form. Certificates should be uploaded at the attachments page.
Additional information
On the Summary/Submit page in the additional information field, list all the countries dog is expected to travel to during its time outside the United States. If the dog has more than one microchip, you can enter the additional microchip information and implant date here.
